Electronic configuration (E.C.) means configuring electron in an
atom.
There are a lots of way from we can do e.c. Of an element.
We can do it by shell wise, subshell or orbit wise or we can do it by orbital
wise or spin wise.
Basically according to
quantum theory, there are four quantum number. The quantum theory is nothing
but it is a complete analysis of structure, shape, orientation, spin and
magnetic property of an element. Here we only focus on our topic, so we only
understand main point of quantum number but not in detail.
ELECTRONIC CONFIGURATION part 2 .
>>>click hereIn the 2nd part we will learn about application or use of electronic configuration. Here we will see how can we know element‘s atomic number, its block and the group and period of that element from the electronic configuration .
1. Principle
quantum number – It is
basically shell or energy level relating quantum number. This quantum number
tells in which shell or energy level electron is present. It is denoted by n
which means no of shell. A shell can contain 2n2 no. Of electron in
it.
For example:
Shell
( n)
|
maximum electron
|
1 or k
|
2
|
2 or l
|
8
|
3 or m
|
18
|
4 or n
|
32
|
2. Azimuthal
quantum number - – it is
basically subshell relating quantum number.it tell about shape of orbitals or
subshell. It is denoted by l (0, 1, 2, and 3).
Subshell
|
Azimuthal quantum number (l)
|
S
|
0
|
P
|
1
|
D
|
2
|
F
|
3
|
the no. Of subshell in a shell can be
determined by value of n and l. This l may have value from
0 to (n-1) for each of principle energy level.
N
|
L to (n-1)
|
Subshells
|
Subshells with n
|
1
|
0 to 0
|
S
|
1s
|
2
|
0 to 1
|
S,p
|
2s, 2p
|
3
|
0 to 2
|
S,p,d
|
3s, 3p, 3d
|
4
|
0 to 3
|
S,p,d,f
|
4s, 4p, 4d, 4f
|
3. Magnetic
quantum numbers – it is
related to orientation of subshell. These different orientations are called
orbital. It is denoted by ml.
We can find no of orbital and
electron present in a subshell with the help of l. These calculations can be
done by (2l+1).
Subshell
|
No. Of orbitals (2l+1)
|
No. Of electron (2ml)
|
S
|
1
|
2
|
P
|
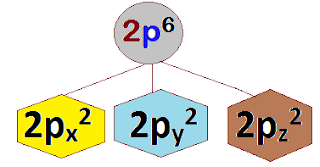
3 (px ,py, pz)
|
6
|
D
|
5 (dxy, dyz, dxz, dx2-y2,
dz2 )
|
10
|
F
|
7
|
14
|
4. Spin
quantum number- it tells
about spins of electron in an orbital of an atom. It is denoted by ms. The spin of electron is denoted by arrow sign
(↑,↓) . One arrow denotes clockwise spin and other
denotes anti- clock wise spin.
Now ,we
understand the rules of configuration.
1) Bohr-bury scheme- This rule decide which orbital have more energy.
a.
If
a subshell has lower value of (n+l) , that subshell having low energy.
Ex:- 4s=(4+0)=4 ,3d=(3+2)=5 ; here 3d has higher energy
than 4s .
b.
If value of (n+l) is same for two subshell then
lower value of n has lower energy.
ex;- 4s=(4+0)=4,3p=(3+1)=4; here 4s
has higher energy than 3p.
2) Aufbau rule-
According to this rule electrons are filled in a orbital on the basis of their
respective energy. That means , electron having lower energy fills first in an
orbital then electron having greater energy
fills.
3) Pauli
exclusion principle- This principle states that no two electron in an atom
can have same set of all four quantum number.
4) Hund’s
rule- According to this rule in p, d ,f orbital ,the electrone will
not pair up until all orbital not having atleast one electron.
5) Last
shell of an atom can have maximum number of 8 electrons.
So, lets start e.c. of an atom
Ex: mg ( magnesium)
Atomic number =12Electronic configuration : 1s2 2s2 2p6 3s2 (this is subshell wise configuration)
# No of electrons present in shell(1,2,3,4)
n
|
n
|
subshell
|
Electrons
in shell
|
1
|
K
|
1s
|
2
|
2
|
L
|
2s 2p
|
8
|
3
|
M
|
3s 3p
3d
|
18
|
4
|
N
|
4s 4p
4d 4f
|
32
|
IMPORTANT QUESTION FROM ELECTRONIC CONFIGURATION
EXCEPTION:
24- CHROMIUM and 29-COPPER has unique e.c..
A half filled and full filled orbital is more stable than less than half filled or less than full filled orbital.So, 24-Cr and 29-Cu want to achieve half filled and full filled D orbital respectively. so 24-Cr and 29-Cu complete its half filled and full filled D orbital by borrowing a electron from 4s orbital.
Please use desktop mode or rotate your device to read comfortably if you have smartphone.
Click here to see image of period table
ELECTRONIC CONFIGURATION
|
|||||||||
z
|
symbol
|
Electronic
configuration ( subshell wise)
|
n
(SHELL WISE)
|
||||||
1
|
2
|
3
|
4
|
5
|
6
|
7
|
|||
1
|
H
|
1s1
|
1
|
||||||
2
|
He
|
1s2
|
2
|
||||||
3
|
Li
|
1s2 2s1
|
2
|
1
|
|||||
4
|
Be
|
1s2 2s2
|
2
|
2
|
|||||
5
|
B
|
1s2 2s2
2p1
|
2
|
3
|
|||||
6
|
C
|
1s2 2s2
2p2
|
2
|
4
|
|||||
7
|
N
|
1s2 2s2
2p3
|
2
|
5
|
|||||
8
|
O
|
1s2 2s2
2p4
|
2
|
6
|
|||||
9
|
Fe
|
1s2 2s2
2p5
|
2
|
7
|
|||||
10
|
Ne
|
1s2 2s2
2p6
|
2
|
8
|
|||||
11
|
Na
|
1s2 2s2
2p6 3s1
|
2
|
8
|
1
|
||||
12
|
Mg
|
1s2 2s2
2p6 3s2
|
2
|
8
|
2
|
||||
13
|
Al
|
1s2 2s2
2p6 3s2 3p1
|
2
|
8
|
3
|
||||
14
|
Si
|
1s2 2s2
2p6 3s2 3p2
|
2
|
8
|
4
|
||||
15
|
P
|
1s2 2s2
2p6 3s2 3p3
|
2
|
8
|
5
|
||||
16
|
S
|
1s2 2s2
2p6 3s2 3p4
|
2
|
8
|
6
|
||||
17
|
Cl
|
1s2 2s2
2p6 3s2 3p5
|
2
|
8
|
7
|
||||
18
|
Ar
|
1s2 2s2
2p6 3s2 3p6
|
2
|
8
|
8
|
||||
19
|
K
|
1s2 2s2
2p6 3s2 3p6 4s1 or
Ar 4s1
|
2
|
8
|
8
|
1
|
|||
20
|
Ca
|
1s2 2s2
2p6 3s2 3p6 4s2
or Ar 4s2
|
2
|
8
|
8
|
2
|
|||
21
|
Sc
|
Ar 4s2 3d1
|
2
|
8
|
9
|
2
|
|||
22
|
Ti
|
Ar 4s2 3d2
|
2
|
8
|
10
|
2
|
|||
23
|
V
|
Ar 4s2
3d3
|
2
|
8
|
11
|
2
|
|||
24
|
Cr
|
Ar 4s1
3d5
|
2
|
8
|
13
|
1
|
|||
25
|
Mn
|
Ar 4s2
3d5
|
2
|
8
|
13
|
2
|
|||
26
|
Fe
|
Ar 4s2
3d6
|
2
|
8
|
14
|
2
|
|||
27
|
Co
|
Ar 4s2
3d7
|
2
|
8
|
15
|
2
|
|||
28
|
Ni
|
Ar 4s2
3d8
|
2
|
8
|
16
|
2
|
|||
29
|
Cu
|
Ar 4s1
3d10
|
2
|
8
|
18
|
1
|
|||
30
|
Zn
|
Ar 4s2
3d10
|
2
|
8
|
18
|
2
|
|||
31
|
Ga
|
Ar 4s2
3d10 4p1
|
2
|
8
|
18
|
3
|
|||
32
|
Ge
|
Ar 4s2
3d10 4p2
|
2
|
8
|
18
|
4
|
|||
33
|
As
|
Ar 4s2
3d10 4p3
|
2
|
8
|
18
|
5
|
|||
34
|
Se
|
Ar 4s2
3d10 4p4
|
2
|
8
|
18
|
6
|
|||
35
|
Br
|
Ar 4s2
3d10 4p5
|
2
|
8
|
18
|
7
|
|||
36
|
Kr
|
Ar 4s2
3d10 4p6
|
2
|
8
|
18
|
8
|
|||
37
|
Rb
|
Kr
5s1
|
2
|
8
|
18
|
8
|
1
|
||
38
|
Sr
|
Kr
5s2
|
2
|
8
|
18
|
8
|
2
|
||
39
|
Y
|
Kr
4d1 5s2
|
2
|
8
|
18
|
9
|
2
|
||
40
|
Zr
|
Kr
4d2 5s2
|
2
|
8
|
18
|
10
|
2
|
||
41
|
Nb
|
Kr
4d4 5s1
|
2
|
8
|
18
|
12
|
1
|
||
42
|
Mo
|
Kr
4d5 5s1
|
2
|
8
|
18
|
13
|
1
|
||
43
|
Tc
|
Kr
4d5 5s2
|
2
|
8
|
18
|
13
|
2
|
||
44
|
Ru
|
Kr
4d7 5s1
|
2
|
8
|
18
|
15
|
1
|
||
45
|
Rh
|
Kr
4d8 5s1
|
2
|
8
|
18
|
16
|
1
|
||
46
|
Pd
|
Kr
4d10
|
2
|
8
|
18
|
18
|
|||
47
|
Ag
|
Kr
4d10 5s1
|
2
|
8
|
18
|
18
|
1
|
||
48
|
Cd
|
Kr
4d10 5s2
|
2
|
8
|
18
|
18
|
2
|
||
49
|
In
|
Kr
4d10 5s2 5p1
|
2
|
8
|
18
|
18
|
3
|
||
50
|
Sn
|
Kr
4d10 5s2 5p2
|
2
|
8
|
18
|
18
|
4
|
||
51
|
Sb
|
Kr
4d10 5s2 5p3
|
2
|
8
|
18
|
18
|
5
|
||
52
|
Te
|
Kr
4d10 5s2 5p4
|
2
|
8
|
18
|
18
|
6
|
||
53
|
I
|
Kr
4d10 5s2 5p5
|
2
|
8
|
18
|
18
|
7
|
||
54
|
Xe
|
Kr
4d10 5s2 5p6
|
2
|
8
|
18
|
18
|
8
|
||
55
|
Cs
|
Xe
6s1
|
2
|
8
|
18
|
18
|
8
|
1
|
|
56
|
Ba
|
Xe
6s2
|
2
|
8
|
18
|
18
|
8
|
2
|
|
Lanthanide
series (57-71)
|
|||||||||
57
|
La
|
Xe 6s2 5d1
|
2
|
8
|
18
|
18
|
9
|
2
|
|
58
|
Ce
|
Xe
4f2 6s2
|
2
|
8
|
18
|
20
|
8
|
2
|
|
59
|
Pr
|
Xe
4f3 6s2
|
2
|
8
|
18
|
21
|
8
|
2
|
|
60
|
Nd
|
Xe
4f4 6s2
|
2
|
8
|
18
|
22
|
8
|
2
|
|
61
|
Pm
|
Xe
4f5 6s2
|
2
|
8
|
18
|
23
|
8
|
2
|
|
62
|
Sm
|
Xe
4f6 6s2
|
2
|
8
|
18
|
24
|
8
|
2
|
|
63
|
Eu
|
Xe
4f7 6s2
|
2
|
8
|
18
|
25
|
8
|
2
|
|
64
|
Gd
|
Xe
4f7 5d1 6s2
|
2
|
8
|
18
|
25
|
9
|
2
|
|
65
|
Tb
|
Xe
4f9 6s2
|
2
|
8
|
18
|
27
|
8
|
2
|
|
66
|
Dy
|
Xe
4f10 6s2
|
2
|
8
|
18
|
28
|
8
|
2
|
|
67
|
Ho
|
Xe
4f11 6s2
|
2
|
8
|
18
|
29
|
8
|
2
|
|
68
|
Er
|
Xe
4f12 6s2
|
2
|
8
|
18
|
30
|
8
|
2
|
|
69
|
Tm
|
Xe
4f13 6s2
|
2
|
8
|
18
|
31
|
8
|
2
|
|
70
|
Yb
|
Xe
4f14 6s2
|
2
|
8
|
18
|
32
|
8
|
2
|
|
71
|
Lu
|
Xe
4f14 5d1 6s2
|
2
|
8
|
18
|
32
|
9
|
2
|
|
72
|
Hf
|
Xe
4f14 5d2 6s2
|
2
|
8
|
18
|
32
|
10
|
2
|
|
73
|
Ta
|
Xe
4f14 5d3 6s2
|
2
|
8
|
18
|
32
|
11
|
2
|
|
74
|
W
|
Xe
4f14 5d4 6s2
|
2
|
8
|
18
|
32
|
12
|
2
|
|
75
|
Re
|
Xe
4f14 5d5 6s2
|
2
|
8
|
18
|
32
|
13
|
2
|
|
76
|
Os
|
Xe
4f14 5d6 6s2
|
2
|
8
|
18
|
32
|
14
|
2
|
|
77
|
Ir
|
Xe
4f14 5d7 6s2
|
2
|
8
|
18
|
32
|
15
|
2
|
|
78
|
Pt
|
Xe
4f14 5d9 6s1
|
2
|
8
|
18
|
32
|
17
|
1
|
|
79
|
Au
|
Xe
4f14 5d10 6s1
|
2
|
8
|
18
|
32
|
18
|
1
|
|
80
|
Hg
|
Xe
4f14 5d10 6s2
|
2
|
8
|
18
|
32
|
18
|
2
|
|
81
|
Ti
|
Xe
4f14 5d10 6s2 6p1
|
2
|
8
|
18
|
32
|
18
|
3
|
|
82
|
Pb
|
Xe
4f14 5d10 6s2 6p2
|
2
|
8
|
18
|
32
|
18
|
4
|
|
83
|
Bi
|
Xe
4f14 5d10 6s2 6p3
|
2
|
8
|
18
|
32
|
18
|
5
|
|
84
|
Po
|
Xe
4f14 5d10 6s2 6p4
|
2
|
8
|
18
|
32
|
18
|
6
|
|
85
|
At
|
Xe
4f14 5d10 6s2 6p5
|
2
|
8
|
18
|
32
|
18
|
7
|
|
86
|
Rn
|
Xe
4f14 5d10 6s2 6p6
|
2
|
8
|
18
|
32
|
18
|
8
|
|
87
|
Fr
|
Rn
7s1
|
2
|
8
|
18
|
32
|
18
|
8
|
1
|
88
|
Ra
|
Rn
7s2
|
2
|
8
|
18
|
32
|
18
|
8
|
2
|
Actinides
series (89-103)
|
|||||||||
89
|
Ac
|
Rn
6d1 7s2
|
2
|
8
|
18
|
32
|
18
|
9
|
2
|
90
|
Th
|
Rn
6d2 7s2
|
2
|
8
|
18
|
32
|
18
|
10
|
2
|
91
|
Pa
|
Rn
5f2 6d1 7s2
|
2
|
8
|
18
|
32
|
20
|
9
|
2
|
92
|
U
|
Rn
5f3 6d1 7s2
|
2
|
8
|
18
|
32
|
21
|
9
|
2
|
93
|
Np
|
Rn
5f4 6d1 7s2
|
2
|
8
|
18
|
32
|
22
|
9
|
2
|
94
|
Pu
|
Rn
5f6 7s2
|
2
|
8
|
18
|
32
|
24
|
8
|
2
|
95
|
Am
|
Rn
5f7 7s2
|
2
|
8
|
18
|
32
|
25
|
8
|
2
|
96
|
Cm
|
Rn
5f7 6d1 7s2
|
2
|
8
|
18
|
32
|
25
|
9
|
2
|
97
|
Bk
|
Rn
5f9 7s2
|
2
|
8
|
18
|
32
|
27
|
8
|
2
|
98
|
Cf
|
Rn
5f10 7s2
|
2
|
8
|
18
|
32
|
28
|
8
|
2
|
99
|
Es
|
Rn
5f11 7s2
|
2
|
8
|
18
|
32
|
29
|
8
|
2
|
100
|
Fm
|
Rn
5f12 7s2
|
2
|
8
|
18
|
32
|
30
|
8
|
2
|
101
|
Md
|
Rn
5f13 7s2
|
2
|
8
|
18
|
32
|
31
|
8
|
2
|
102
|
No
|
Rn
5f14 7s2
|
2
|
8
|
18
|
32
|
32
|
8
|
2
|
103
|
Lr
|
Rn
5f14 6d1 7s2
|
2
|
8
|
18
|
32
|
32
|
9
|
2
|
104
|
Rf
|
Rn
5f14 6d2 7s2
|
2
|
8
|
18
|
32
|
32
|
10
|
2
|
105
|
Db
|
Rn
5f14 6d3 7s2
|
2
|
8
|
18
|
32
|
32
|
11
|
2
|
106
|
Sg
|
Rn
5f14 6d4 7s2
|
2
|
8
|
18
|
32
|
32
|
12
|
2
|
107
|
Bh
|
Rn
5f14 6d5 7s2
|
2
|
8
|
18
|
32
|
32
|
13
|
2
|
108
|
hs
|
Rn
5f14 6d6 7s2
|
2
|
8
|
18
|
32
|
32
|
14
|
2
|
For any question , comment below.










No comments:
Post a Comment